- #1
WMDhamnekar
MHB
- 376
- 28
Hi,
If any member knows the correct answer to the following question on stoichiometry, may reply to this question.
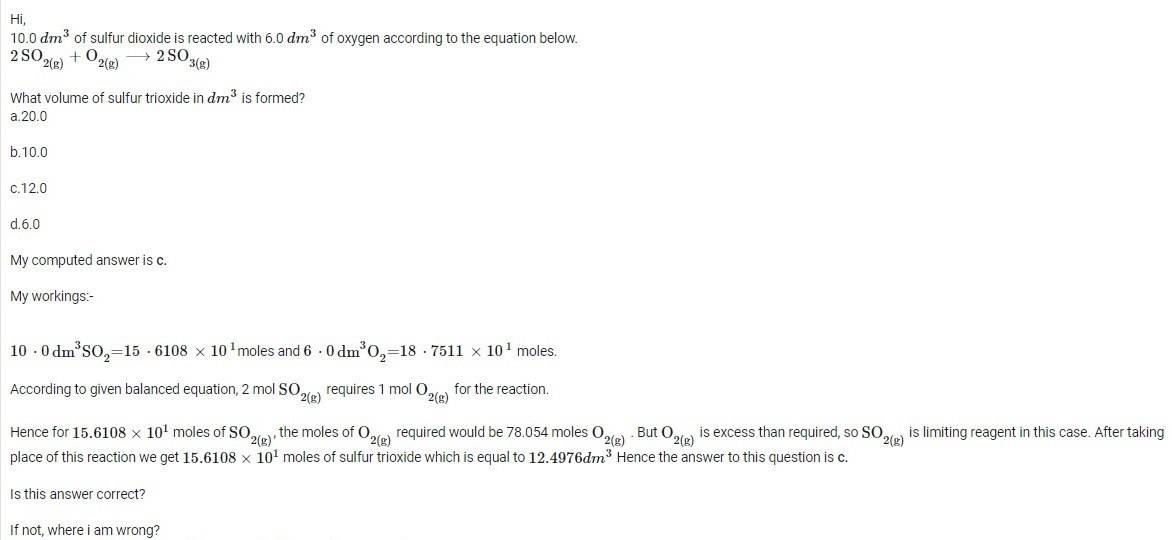
If any member knows the correct answer to the following question on stoichiometry, may reply to this question.
Last edited by a moderator: