- #1
chemistryfourm
- 1
- 0
- TL;DR Summary
- My NMR of diethyl cyclopent-3-ene, 1, 1 , dicarboxylate shows the cyclic proton environments both as singlets where they are expected to be a doublet and triplet
My NMR of diethyl cyclopent-3-ene, 1, 1 , dicarboxylate shows the cyclic proton environments both as singlets where they are expected to be a doublet and triplet.
Proton env B would be epxected to be a doublet and D to be a doublet. Everyone in the lab got the same result. Is there an explanation as to why it they are singlets and not multiplets as expected? The chemical shift and integration suggest they are the expected proton enviornment however the multiplicity is not as expected.
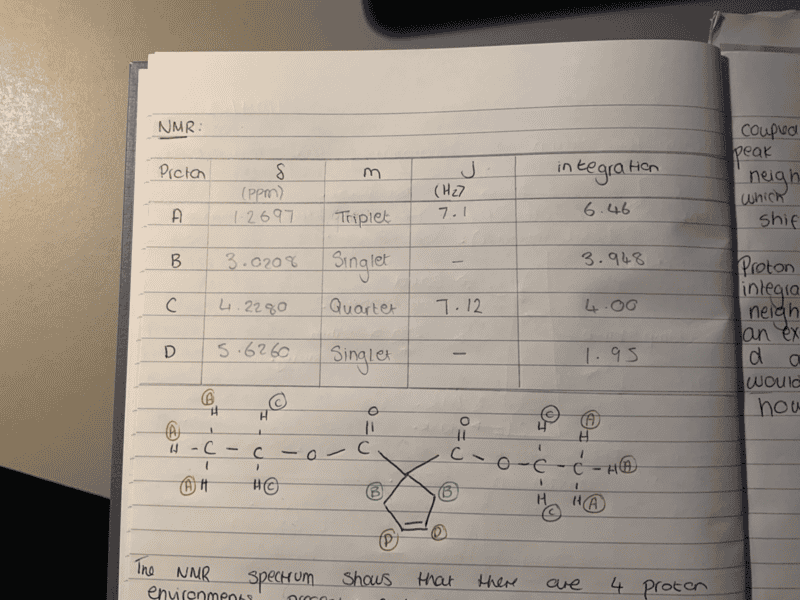
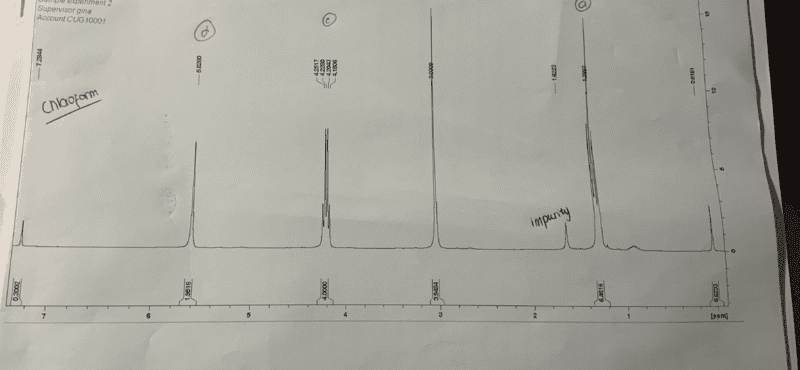
Proton env B would be epxected to be a doublet and D to be a doublet. Everyone in the lab got the same result. Is there an explanation as to why it they are singlets and not multiplets as expected? The chemical shift and integration suggest they are the expected proton enviornment however the multiplicity is not as expected.