- #1
Say17
- 11
- 1
- Homework Statement
- From the following list, indicate the condition(s) that will enable you to obtain at least 27 g of water.
- Relevant Equations
- H2O = 2*1.01 + 16 = 18.02
Hi everyone,
I just don't get it how to compute the following task. First I have to find out many moles are in 27g of Water.
H2O = 2*1.01 + 16 = 18.02
27g/18.02 = 1.5mol but afterwards how do I find each elements gram?
d is the correct answer.
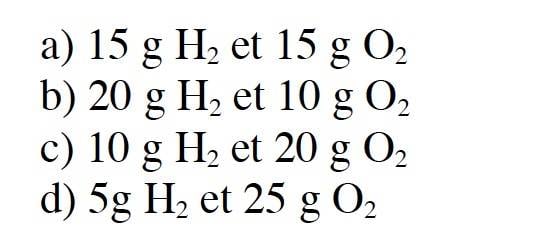
I just don't get it how to compute the following task. First I have to find out many moles are in 27g of Water.
H2O = 2*1.01 + 16 = 18.02
27g/18.02 = 1.5mol but afterwards how do I find each elements gram?
d is the correct answer.